- Home
- What we do
- Market
- MedTech
- Medical Imaging
MEDICAL IMAGING
Advanced imaging solutions for better care outcomes
Harness the power of cloud, AI, and digital engineering to drive transformation, design efficient workflows and improve outcomes
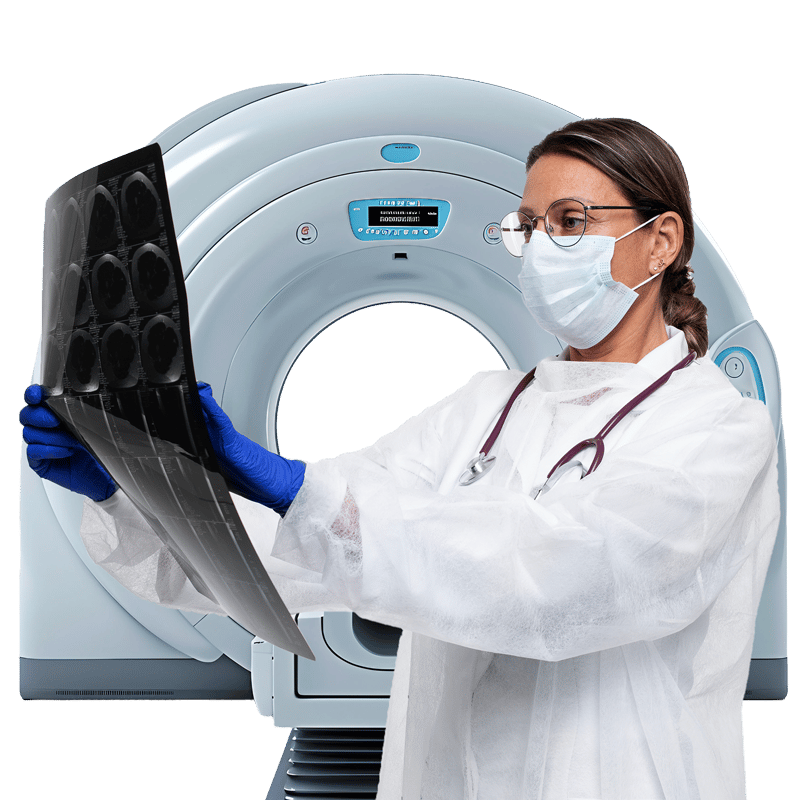
MEDICAL IMAGING
Implementing AI-powered cloud imaging solutions
Manage data growth and radiologist workload, build collaborations, and integrate new data sources for better patient insights with CitiusTech’s AI and cloud technology.
Custom visualization workflow
Multi-modality image acquisition
Quality and regulatory compliance
Real-time 3D rendering
Interoperability and data management expertise
AI/ML-enabled image post-processing
WHY CITIUSTECH
Redefining imaging performance
+
Imaging engagements
across different sizes and complexities
+
Imaging and
implementation
experts
+
Imaging experts with
over 15 years of
experience
+
Imaging industry
luminaries are our
advisors
+
Imaging organizations, including the top five imaging ISVs in the world, leverage our solutions
features
Revolutionizing medical imaging & data management
Discover advanced visualization
Build a custom visualization workflow providing product development, real-time 3D rendering & UX modernization for thick & thin client viewers.
Enhance interoperability and data management
Integrate workflow, clinical decision-making, and patient care coordination with interoperability, including data aggregation and cloud migration.
Access multi-modality images
Acquire interoperable data through console applications for various modalities like CT, MRI, USG, PET-CT, X-ray, and endoscopy.
Achieve workflow optimization
Speed track and manage medical images with our implementation and support services of end-to-end protocol management and seamless workflow.
Operationalize AI-driven imaging
Gain faster access to images and patient metadata with AI-driven worklist prioritization, image pattern recognition, ROI identification, and GenAI-enabled chatbots.
Modernize radiology productivity
Boost efficiency by updating RIS/PACS/VNA for efficient data aggregation. Set up seamless data access through de-identification and integration of third-party applications.

SUCCESS STORIES
Solving some of the greatest challenges in imaging

Case Study

Case Study

Case Study
SERVICES
Shaping Healthcare possibilities with our expertise
%20(3).webp)



Digital Engineering
Reshaping Healthcare with cutting-edge technology solutions for better patient care

Data Engineering & Data on Cloud
Making Healthcare data the backbone of your organization's strategy


Explore other Services
Harness deep domain expertise and customized digital solutions to craft a seamless and integrated healthcare experience. With CitiusTech, you can also bridge the gap between healthcare and technology, fostering a healthier future.






.png?width=1920&height=1080&name=Consulting2_Menu_1%20(1).png)


















